819
Views & Citations10
Likes & Shares
Idiopathic intracranial hypertension, earlier also known as
pseudotumor cerebri (PTC), is a rare idiopathic disease classically manifesting
with headache in obese women. It is characterized by raised intracranial
pressure (ICP) with normal CSF composition and absence of hydrocephalus or
intracranial space occupying lesions. The hallmark of IIH is papilledema, which
may be bilateral, asymmetrical or even unilateral; however, IIH can occur in
the absence of papilledema. The diagnosis of IIH is, therefore, not always
simply achieved.
Keywords: Idiopathic, Hypertension, Pseudotumor cerebri, Intracranial pressure, Papilledema
INTRODUCTION
IIH is a headache syndrome characterized by raised CSF
pressure in the absence of any intracranial lesion or other underlying systemic
cause. The term “PTC”
was coined in 1904 by Nonne to describe a condition characterized by symptoms
associated with intracranial tumors with an unusual course of remission and
subsequently termed “benign intracranial hypertension” by Foley in 1955 [1].
Heinrich Quincke, an early pioneer in the use of
lumbar puncture, reported the first recorded cases of intracranial hypertension
of unknown cause in what he described as “meningitis serosa” in 1893; at that
time, he postulated that inadequate CSF resorption was responsible for the
syndrome, a theory that is still entertained by some researchers [2].
Most of the cases (90%) are idiopathic in origin, but
in some there exists a secondary cause. Therefore, many authors prefer the term
Pseudotumor cerebri over IIH, which includes both: purely IIH and that due to
secondary causes of intracranial hypertension such as venous stenosis [3].
ETIOLOGY
As the name suggests,
etiology is largely unknown and is mainly idiopathic. There are few
associations which are reported as causing raised intracranial hypertension.
These are listed in table below:
Associations that have been reported as causing raised intracranial pressure [4,5]
|
Hematological |
Anemia, Polycythemia vera |
|
Obstruction to venous
drainage |
Cerebral venous sinus thrombosis |
|
Jugular vein thrombosis |
|
|
Superior vena cava syndrome |
|
|
Jugular vein ligation following bilateral radical neck dissection |
|
|
Increased right heart pressure |
|
|
Arteriovenous fistulas |
|
|
Previous infection or subarachnoid hemorrhage causing decreased
CSF absorption |
|
Medications |
Lithium |
|
Vitamin A derivatives (including isotretinoin and
all-transretinoic acid) |
|
|
Nalidixic acid |
|
|
Danazol |
|
|
Tetracycline class antibiotics |
|
|
Corticosteroid withdrawal |
|
|
Levothyroxine |
|
|
Tamoxifen |
|
|
Ciclosporin |
|
|
Levonorgestrel implant |
|
|
Fluoroquinolones |
|
|
Growth hormone |
|
|
Indomethacin |
|
|
Cimetidine |
|
|
Systemic
disorders |
Chronic kidney disease/renal failure |
|
Obstructive sleep apnoea syndrome |
|
|
Chronic obstructive pulmonary disease |
|
|
Systemic lupus erythematosus |
|
|
Psittacosis |
|
|
Endocrine |
Addison’s disease |
|
Cushing’s syndrome |
|
|
Hypoparathyroidism |
|
|
Hypothyroidism |
|
|
Hyperthyroidism |
|
|
Syndromic |
Down syndrome |
|
Craniosynostosis |
|
|
Turner syndrome |
PRESENTATION
There incidence of IIH peaks in third
decade of life. It most frequently occurs in obese females of childbearing age
but can occur in all age groups, both genders and both obese and non-obese
individuals. The condition is infrequent in children (in whom obesity is less a
factor), men and lean adults [5].
PTC classically presents
with headache and, frequently, vision changes in women with obesity of
childbearing age. Headaches occur in nearly all (90%-94%) patients with
PTC—they are characteristically pressure like, throbbing, and usually
unremitting and occur with retro-ocular pain and may be accompanied by nausea
or vomiting.
Headache
attributed to IIH, as described by the International Classification of Headache
Disorders, 3rd edition (beta version) (ICHD-3 beta) [6].
a)
IIH diagnosed by lumbar puncture
opening pressure of >25 cm H2O.
b)
Evidence of causation demonstrated
by two of following:
i.
Headache developed in temporal
relation to IIH.
ii.
Headache relieved by reducing ICP.
iii.
Headache exacerbated in temporal
relationship to increased ICP.
c)
Headache not accounted for by
another ICHD-3 diagnosis.
Other symptoms, in order of frequency,
reported by Markey et al. [7] include:
·
Visual
obscuration (darkening of vision) (68-72%)
·
Pulsatile
tinnitus (52-61%)
·
Back
pain (53%)
·
Dizziness
(52%)
·
Neck
pain (42%)
·
Blurred
vision (32%)
·
Cognitive
disorder (20%)
·
Radicular
pain (19%)
·
Diplopia,
typically horizontal (18%)
Vision loss is the most feared sequel of
PTC, thought to be related to ischemia of optic nerve due to increased CSF
pressure. Other reason for loss of vision is raised IOP which directly
correlates with ICP [8] due to direct anatomic connection between cranial fossa
and orbit. Mostly vision loss in this syndrome is transient in nature, less
frequently, it takes the form of impairments in the visual field, directly
correlated with the extent of disc edema, with the typical impairment
presenting as tunnel vision. Diplopia is usually horizontal in nature due to
involvement of sixth nerve by raised ICP. Other common symptoms include
photopsia and eye pain.
Ophthalmologic signs of PTC consist of
diminished visual acuity, visual field losses in nearly all patients, and, most
strikingly, papilledema on fundus examination in 40% of patients. Absence of
papilledema has been reported in many populations of patients with IIH, but its
absence may be more suggestive of an alternative etiology for headache and
vision loss [5]. Other funduscopic findings that may be seen in PTC are
choroidal folds, parallel striae of alternating yellow crests and darker
troughs; choroidal folds compromise vision and can be seen with elevated ICP,
even when papilledema has resolved [9,10].
Cranial nerve palsies, usually of the
abducens nerve (CN VI) Rarely, facial nerve (CN VII) palsies may be associated
with IIH [11].
Tinnitus, pulse-synchronous is another commonly
reported symptom of PTC and is often described as a unilateral “whooshing”
sound by patients and may be exacerbated by positional changes and relieved by
jugular compression [5,11,12].
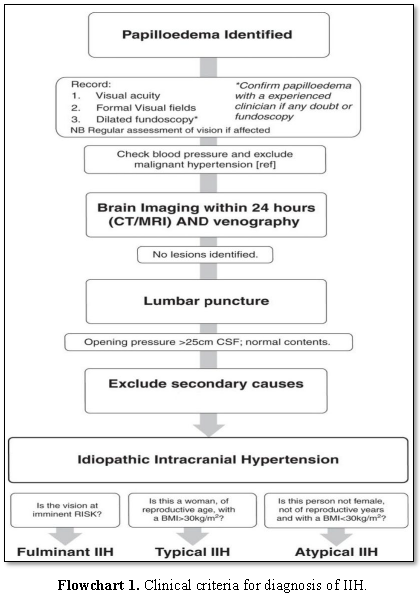
DIAGNOSIS
Clinical criteria for diagnosis
of IIH (Adapted from Friedman and Jacobson) [13]:
·
Symptoms
and signs attributed to increased ICP.
·
Documented
elevated ICP during lumbar puncture with manometry, typically >25 cm H2O
in adults and >28 cm H2O in children, measured in the lateral
decubitus position with legs and head in a straight and relaxed position
·
Normal
cerebrospinal fluid composition (normal cell count, normal glucose, normal
protein)
·
No
evidence of ventriculomegaly, mass, structural or vascular lesion on magnetic
resonance imaging or contrast enhanced computer tomography and normal magnetic
resonance venography imaging.
·
Normal
neurological examination, with exception that patient may have a sixth nerve
palsy.
Direct transmission of the elevated CSF
pressure results in distension of the perioptic subarachnoid space and
ballooning of the optic papilla, causing it to protrude physically into the
posterior aspect of the globe [14-17]. The long-standing effect of pulsatile
CSF under high pressure also leads to downward herniation of an arachnocele
through a defect in the diaphragm sella (Flowchart
1) [18].
MR imaging of the optic nerves and
pituitary gland may provide important clues for the diagnosis of IIH, with a return
to normal appearance after normalization of CSF pressure. The use of
high-resolution, thin-slice MR imaging improves the visualization of the optic
nerves and pituitary gland. Characteristic MRI findings include [19].
·
Flattening
of globes
·
Partially
empty sella
·
Narrowing
of the distal transverse venous sinus
·
Distension
of the perioptic subarachnoid space
·
Enhancement
of the prelaminar optic nerve
·
Vertical
tortuosity of the orbital optic nerve
·
Intraocular
protrusion of the prelaminar optic nerve
Patient with suspected elevated
intracranial hypertension must also undergo MR venography in addition to
traditional MR orbital imaging to evaluate venous thrombosis or stenosis as the
etiology of PTC symptoms.
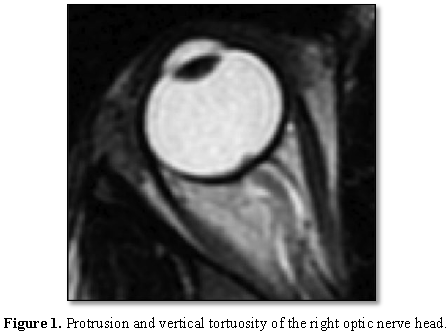
Protrusion of the right optic nerve head and
vertical tortuosity of the optic nerve are seen in this 21 year old woman on
axial T2-weighted MR imaging (Figure 1).
Clinically, the patient presented with headaches, vision changes and
papilledema noted on examination [19].
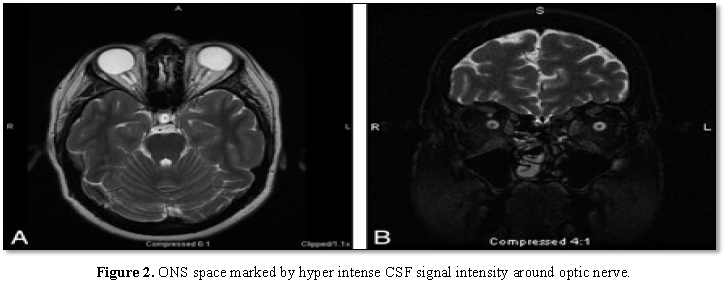
The Optic Nerve Sheath (ONS) is widened
with expanded CSF hyper intensity surrounding the optic nerve, seen on axial
T2-weighted MR imaging in conjunction with posterior flattening of the globes.
ONS widening is thought to coincide with papilledema, which is seen in this 27 year
old woman who presented with headaches (Figure
2A). Coronal T2-weighted MR imaging in a 55 year old woman with headache
demonstrates increased peri-ONS space marked by hyper intense signal intensity
surrounding the optic nerve [19] (Figure
2B).
FOLLOW UP
The ophthalmologist plays a crucial role in
the management of IIH. Careful long-term follow up of vision and papilloedema
is necessary. Regular examination should include testing of visual acuity,
color vision, quantitative perimetery and photograph of optic nerve head.
Repeat OCT can also be used but not in isolation to follow papilledema because
secondary optic atrophy from untreated papilledema will also result in apparent
improvement of the RNFL thickness on OCT. However, it may be differentiated by
the ganglion complex at the macula. The frequency of visual field testing
depends on the severity of papilledema, the level of optic nerve dysfunction
and the patient response to treatment.
Zagardo et al. [20] found that the
previously compressed pituitary gland had re expanded to fill the sella turcica
after normalization of CSF pressure. This suggests that acute or sub-acute
elevation of CSF pressure may be sufficient to compress the pituitary gland.
Repeat MR imaging of the present patient also showed reversibility of a
partially empty sella and normalization of the volume of the optic nerve
sheaths, which had not been previously reported. The return to a normal
appearance of the pituitary gland and optic nerves on MR images may indicate a
positive response to therapy and possibly denote a corresponding decrease in
CSF pressure.
TREATMENT
The main goals of treatment are alleviation
of symptoms and preservation of vision. The approach used in a particular
patient depends on the severity and time course of their symptoms and visual
loss, as determined by perimetry. Obese patients should be encouraged to lose a
modest amount of weight. Potential contributing factors (e.g. obstructive sleep
apnea) should be treated.
DIET
AND LIFESTYLE
In patients with minimal symptoms, signs
and visual loss, weight management program with a low-salt diet and lifestyle
changes, including an exercise program, is a reasonable initial treatment
strategy. A recent prospective study of obese IIH patients found that weight
loss leads to reduced symptoms, signs and ICP [21].
MEDICATION
Pharmacologic treatments can be considered
for patients with mild to moderate disease.
Acetazolamide
The IIH Treatment Trial reported the use of
acetazolamide with a low-sodium weight-reduction diet compared with diet alone
resulted in modest improvement in visual field function in patients with mild
visual loss [22]. It acts by decreasing CSF production and thereby decreasing
ICP.
Dosage: No standardized dose is available. A
reasonable starting dosage is 500 mg twice daily. It can be increased up to 4 g
daily divided in two dosages.
Contraindications: Known hypersensitivity, including sulfa
allergy, liver failure.
It is also relatively contraindicated in
patients with a history of renal stones.
Side
effects: Paresthesias
which may be minimised using potassium supplements, altered taste sensation,
lethargy, drowsiness, anorexia and metabolic acidosis.
Topiramate and furosemide can be considered
when acetazolamide is poorly tolerated or insufficient.
Topiramate has carbonic anhydrase activity
and can suppress appetite. It has been favorably compared with acetazolamide in
an uncontrolled open label study for IIH [23].
·
There
may be a role for topiramate in IIH with weekly dose escalation from 25 mg to
50 mg bd.
·
Where
topiramate is prescribed, women must be informed that it can reduce the efficacy
of the contraceptive pill/oral contraceptives and other hormonal
contraceptives.
·
When
topiramate is prescribed, women must be counselled regarding side effects
(including depression and cognitive slowing) and potential teratogenetic risks.
Since angle-closure glaucoma can sometimes
develop with topiramate treatment, patients who develop eye pain, eye redness
and changes in vision should seek an immediate ophthalmic evaluation.
INTERVENTIONAL TREATMENT
Lumbar
puncture
IIH symptoms (e.g. headache) often improve
following the diagnostic lumbar puncture. In most cases, the improvement is
transient, but occasional patients can have a lasting remission following a
lumbar puncture [24]. Repeated lumbar punctures have been used for treatment
for IIH, but should no longer be considered standard treatment as they are
often technically difficult and poorly tolerated.
SURGICAL TREATMENT
Optic
nerve sheath fenestration (ONSF) surgery
ONSF is a surgical technique to reduce the
hydrostatic pressure on the ONH by following mechanisms:
a)
Opening
within the optic nerve sheath allow for a sudden and sustained drop in the sub
arachnoid Space (SAS) pressure and relief of the compartment syndrome on the
ONH.
b)
It
creates a CSF filter from the SAS of the optic nerve into the surrounding
orbital tissue, thereby reducing the CSF volume and pressure surrounding the
ONH.
c)
ONSF
is thought to increase the velocity of CSF in the optic nerve sheath and
thereby decrease the CSF pressure transmitted to ONH [25].
d)
ONSF
promotes fibrous tissue proliferation at the incisional site, thereby
preventing the transmission of elevated CSF pressure to the ONH [26,27].
In a recent study, 62 IIH patients with
bilateral papilledema who underwent unilateral ONSF were found to have a
decrease in the median grade of papilledema in both the operated and the
non-operated eye. The median grade of papilledema in the operated eye decreased
from grade 3 preoperatively to grade 0.5 by 12 months. The median grade of
papilledema in the non-operated eye decreased from grade 2 before surgery to a
grade 1, 12 months postoperatively [28].
CONTRAINDICATIONS
Contraindications to ONSF include infection
at the surgical site and anticoagulation use.
COMPLICATIONS
Complications are usually minor if the
surgeon is experienced. A tonic pupil can occur if the ciliary nerves are
damaged. Transient or permanent visual loss can occur if there is trauma to the
optic nerve or its vascular supply.
Cerebrospinal fluid shunting
It causes rapid reduction in ICP and
thereby leads to rapid improvement in symptoms and signs. The two procedures
most commonly performed are lumbo-peritoneal (LP) and ventriculo-peritoneal
(VP) shunting. VP shunting is more difficult and usually requires a
stereotactic approach, as IIH patients do not have enlarged ventricles,
however, it is preferred due to its lower complication rate [29,30]
Contraindications of CSF shunting include:
·
When
there is active infection
·
In
patients taking anticoagulants, due to increased risk of bleeding.
Complications of CSF shunting include:
·
Shunt
infections
·
Shunt
obstruction
·
Migration
of the shunt tubing
·
Shunt
failure
·
Over-shunting
and intracranial hypotension can occasionally occur, but are less common since
the introduction of programmable shunt valves.
SUMMARY AND CONCLUSION
IIH is not an uncommon entity in routine
ophthalmic practice. The diagnosis requires a considerable degree of suspicion
on part of the ophthalmologist. Collaboration between ophthalmologists and
neurologists is vital in the optimal management of this potentially sight
threatening condition. Timely detection and early treatment including life
style modification and adjuvant drug therapy should improve prognosis in
majority of the patients and also avoid the need for surgical procedure in
majority of such patients.
1.
Pearce
JM (2009) From pseudotumor cerebri to idiopathic intracranial hypertension.
Pract Neurol 9: 353-356.
2.
Johnston
I (2001) The historical development of the pseudotumor concept. Neurosurg Focus
11: 1-9.
3.
Sylaja
PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S (2003)
Differential diagnosis of patients with intracranial sinus venous
thrombosis-related isolated intracranial hypertension from those with
idiopathic intracranial hypertension. J Neurol Sci 215: 9-12.
4.
Friedman
DI (1998) Novel.utah.edu Walshand Hoyt Text, Chapter 5. In: Mille N, Newman NJ,
Walsh and Hoyt’s Clinical Neuro-Ophthalmology. 5th Edn. Baltimore,
MD: Williams & Wilkins Ed, pp: 237-291.
5.
Aggarwal
A, Kaur P, Chhabra K, Singh K, Goyal P (2017) Idiopathic IHT in pediatric
patient. Nepal J Ophthalmol 9: 74-78.
6.
Headache
Classification Committee of the International Headache Society (IHS) (2013) The
international classification of headache disorders. 3rd Edn (beta
version). Cephalalgia 33: 629-808.
7.
Markey
KA, Mollan SP, Jensen RH, Sinclair AJ (2016) Understanding idiopathic
intracranial hypertension: mechanisms, management and future directions. Lancet
Neurol 15: 78-91.
8.
Mills
RP, Heijl A, Wall M (1991) The morphology of visual field damage in idiopathic
intracranial hypertension: An anatomic region analysis. In: Mills RP, Heijl A,
eds. Perimetry Update 1990/1991. Amsterdam, the Netherlands: Kugler Publishers,
pp: 20-27.
9.
Griebel
SR, Kosmorsky GS (2000) Choroidal folds associated with increased intracranial pressure.
Am J Ophthalmol 129: 513-516.
10.
Randhawa
S, Van Stavern GP (2008) Idiopathic intracranial hypertension (pseudotumor
cerebri). Curr Opin Ophthalmol 19: 445-453.
11.
Wall M
(2010) Idiopathic intracranial hypertension. Neurol Clin 28: 593-617.
12.
Giuseffi
V, Wall M, Siegel PZ, Rojas PB (1991) Symptoms and disease associations in
idiopathic intracranial hypertension (pseudotumor cerebri): A case-control
study. Neurology 41: 239-244.
13.
Friedman
DI, Jacobson DM (2004) Idiopathic intracranial hypertension. J Neuroophthalmol
24: 138-145.
14.
Soler
D, Cox T, Bullock P, Calver DM, Robinson RO (1998) Diagnosis and management of
benign intracranial hypertension. Arch Dis Child 78: 89-94.
15.
Brodsky
MC, Vaphiades M (1998) Magnetic resonance imaging in pseudotumor cerebri.
Ophthalmology 105: 1686-1693.
16.
Jinkins
JR, Athale S, Xiong L, Yuh WTC, Rothman MI, et al. (1996) MR of optic papilla
protrusion in patients with high intracranial pressure. AJNR Am J Neuroradiol
17: 665-668.
17.
Gass
A, Barker GJ, Riordan-Eva P, MacManus D, Sanders M, et al. (1996) MRI of the
optic nerve in benign intracranial hypertension. Neuroradiology 38: 769-773.
18.
Geoge
AE (1989) Idiopathic intracranial hypertension: Pathogenesis and the role of MR
imaging. Radiology 170: 21-22.
19.
Degnan
AJ, Levy LM (2011) Pseudotumor cerebri: Brief review of clinical syndrome and
imaging findings. AJNR AM J Neuroradiol 32: 1986-1993.
20.
Zagardo
MT, Cali WS, Kelman SE, Rothman MI (1996) Reversible empty sella in idiopathic
intracranial hypertension: An indicator of successful therapy? Am J Neuroradiol
17: 1953-1956.
21.
Sinclair
AJ, Burdon MA, Nightingale PG, Ball AK, Good P, et al. (2010) Low energy diet
and intracranial pressure in women with idiopathic intracranial hypertension: Prospective
cohort study. BMJ 341: c2701.
22.
Wall
M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, et al. (2014) Effect of
acetazolamide on visual function in patients with idiopathic intracranial
hypertension and mild visual loss: The idiopathic intracranial hypertension
treatment trial. JAMA 311: 1641-1651.
23.
Celebisoy
N, Gökçay F, Sirin H, Akyürekli O (2007) Treatment of idiopathic intracranial
hypertension: Topiramate vs. acetazolamide, an open-label study. Acta Neurol
Scand 116: 322-327.
24.
De
Simone R, Marano E, Fiorillo C, Briganti F, Di Salle F, et al. (2005) Sudden
re-opening of collapsed transverse sinuses and longstanding clinical remission
after a single lumbar puncture in a case of idiopathic intracranial
hypertension: Pathogenetic implications. Neurol Sci 25: 342-344.
25.
Seiff
SR, Shah L (1990) Model for the mechanism of optic nerve sheath fenestration.
Arch Ophthalmol 108: 1326-1329.
26.
Tsai
JC, Petrovichm S, Sadun AA (1995) Histopathological and ultrastructural
examination of optic nerve sheath decompression, Br J Ophthalmol 79: 182-185.
27.
Davidson
SI (1970) A surgical approach to plerocephalic disc edema. Trans Ophthalmol Soc
U K 89: 669-690.
28.
Alsuhaibani
AH, Carter KD, Nerad JA, Lee AG (2011) Effect of optic nerve sheath
fenestration on papilledema of the operated and the contralateral non-operated
eyes in idiopathic intracranial hypertension. Ophthalmology 118: 412-414.
29.
Bynke
G, Zemack G, Bynke H, Romner B (2004) Ventriculoperitoneal shunting for
idiopathic intracranial hypertension. Neurology 63: 1314-1316.
30.
McGirt
MJ, Woodworth G, Thomas G, Miller N, Williams M, et al. (2004) Cerebrospinal
fluid shunt placement for pseudotumor cerebri-associated intractable headache: Predictors
of treatment response and an analysis of long-term outcomes. J Neurosurg 101:
627-632.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Spine Diseases
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)